Syphilis, a sexually transmitted disease caused by the bacterium Treponema Pallidum, can have serious health consequences if left untreated. The iCARE Syphilis IgM/IgG Field Test Kit offers a rapid, reliable method for detecting antibodies to this bacterium in human whole blood, serum, or plasma, aiding in the diagnosis of Syphilis. This guide provides a step-by-step procedure on using the WHO PQ Listed Syphilis Rapid Test Card effectively.
Intended Use
The Syphilis IgM/IgG Field Test Kit is designed for healthcare professionals to conduct a preliminary analysis for Syphilis by detecting IgG and IgM antibodies in human specimens. The WHO PQ Listed Syphilis Rapid Test Card serves as a screening tool among high-risk groups, during regular health examinations, and for blood bank field screen tests. Remember, any reactive result must be confirmed with further testing and clinical findings.
Precautions
- The kit is for in vitro diagnostic use only.
- Do not use past the expiration date or if the pouch is damaged.
- Treat all specimens as potentially infectious.
- Use the test only once and dispose of it properly to prevent infection risk.
Kit Contents
Each WHO PQ Listed Syphilis Rapid Test Card includes:
- Test cards in a foil pouch with a desiccant.
- A plastic dropper for specimen application.
- Sample diluent for specimen preparation.
- A safety lancet for finger blood collection.
- An alcohol swab for site disinfection.
- A package insert with detailed instructions.
Note: A timer is required but not provided.
Storage and Stability
Store the kit between 2 and 30°C, and do not open the pouch until ready to conduct the test.
Assay Procedures For Finger Blood Collection:
- Prepare Materials: Bring the WHO PQ Listed Syphilis Anti-TP Rapid Test Card, sample diluent, alcohol swab, safety lancet, and dropper to room temperature.
- Open Pouch: Carefully remove the test card from its sealed pouch.
- Collect Blood: Use the alcohol swab to clean the finger, then use the safety lancet to prick the finger. Collect the blood with the plastic dropper.
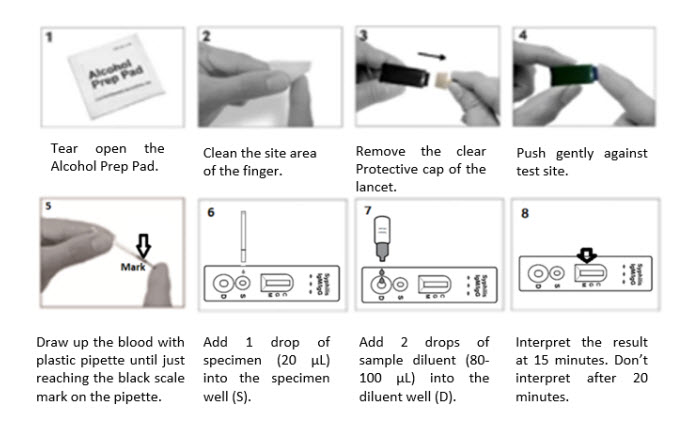
For Clinics (Whole Blood/Serum/Plasma):
- Temperature Equilibration: Allow all reagents and specimens to reach room temperature.
- Test Card Preparation: Open the pouch and place the test card on a clean, dry surface. Label it according to the specimen or control being tested.
- Specimen Application: Using the dropper, add 1 drop of the specimen to the specimen well (S), ensuring no air bubbles. Then, immediately add 2 drops of sample diluent to the diluent well (D).
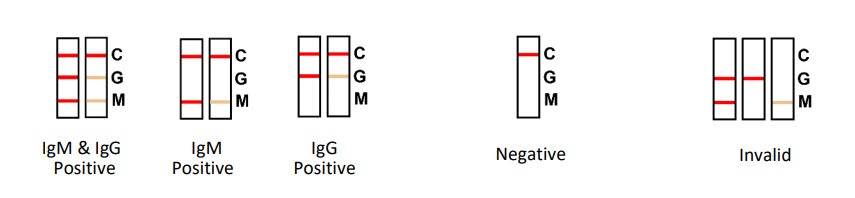
Reading the Test Results (After 15 Minutes)
- Positive: Two or three lines appear. One colored line must be in the control region (C), with additional line(s) in the test line region (G and/or M), indicating a positive result.
- Negative: A single colored line appears in the control region (C) with no line in the test line region. This indicates a negative result for Syphilis antibodies.
- Invalid: If no control line appears, the test is invalid. This could be due to insufficient specimen volume or incorrect procedural techniques. Repeat the test with a WHO PQ Listed Syphilis Anti-TP Rapid Test Card, and if issues persist, discontinue use and contact the distributor.
Conclusion
The Syphilis Test Kit is an essential tool for the rapid screening of Syphilis. By following these steps, healthcare professionals can ensure accurate and reliable preliminary results, which are crucial for the early detection and management of this STD. Remember, positive results require further confirmation through additional testing methods and clinical evaluation.

