Introduction
In light of emerging global health concerns, testing for diseases like Monkeypox has become paramount. The iCARE Monkeypox Virus Antigen Rapid Test Kit is a cutting-edge diagnostic tool designed for the rapid, accurate detection of the Monkeypox virus in various types of human specimens. This kit offers healthcare professionals an efficient solution for in vitro diagnostics, allowing for swift action in managing potential outbreaks and providing critical care.
What is the iCARE Monkeypox Virus Antigen Rapid Test Kit?
The iCARE Monkeypox Virus Antigen Rapid Test Kit is a lateral flow immunoassay designed for the qualitative detection of Monkeypox virus antigens. The kit can be used on various sample types, including whole blood, serum, plasma, oropharyngeal swabs, and exudate of blain (lesion exudate). The rapid test is an essential tool in identifying the presence of Monkeypox antigens to support the early diagnosis of Monkeypox infections.
The Importance of Monkeypox Testing
Monkeypox, a zoonotic orthopoxvirus closely related to smallpox, poses a significant health risk, especially in regions where the virus is endemic, such as central and western Africa. Although the mortality rate of Monkeypox is much lower than that of smallpox, the virus can still cause severe illness in humans. The spread of Monkeypox has also become more prevalent due to factors like international travel and reduced herd immunity against orthopoxviruses. With the iCARE rapid test, healthcare professionals can quickly detect Monkeypox infections and help curb the spread of the virus.
Key Features of the iCARE Monkeypox Virus Antigen Rapid Test Kit
Specimen Versatility
The test can be administered using whole blood, serum, plasma, oropharyngeal swabs, or exudate of blain. This versatility makes it adaptable to different clinical settings, offering flexibility in sample collection.
Fast Results
Results are ready in as little as 15 minutes, making it an efficient option for urgent testing scenarios. The simplicity of the process allows for rapid response in clinical and emergency settings.
High Accuracy
The test kit demonstrates 99.08% sensitivity and 99.67% specificity when compared with PCR testing, the gold standard in virus detection.
Ease of Use
Designed for professional in vitro diagnostic use, the kit is simple to operate, with clear instructions included for healthcare professionals to ensure proper testing.
How Does the Monkeypox Virus Antigen Rapid Test Work?
The iCARE Monkeypox Virus Antigen Rapid Test Kit is based on membrane-based immunoassay technology. During the testing process, a specimen is applied to the test card where it interacts with monoclonal antibodies pre-coated on the membrane. If the Monkeypox virus antigens are present in the specimen, a colored line appears in the test line region (T), indicating a positive result. Regardless of the result, a control line (C) must always appear to confirm that the test was conducted correctly.

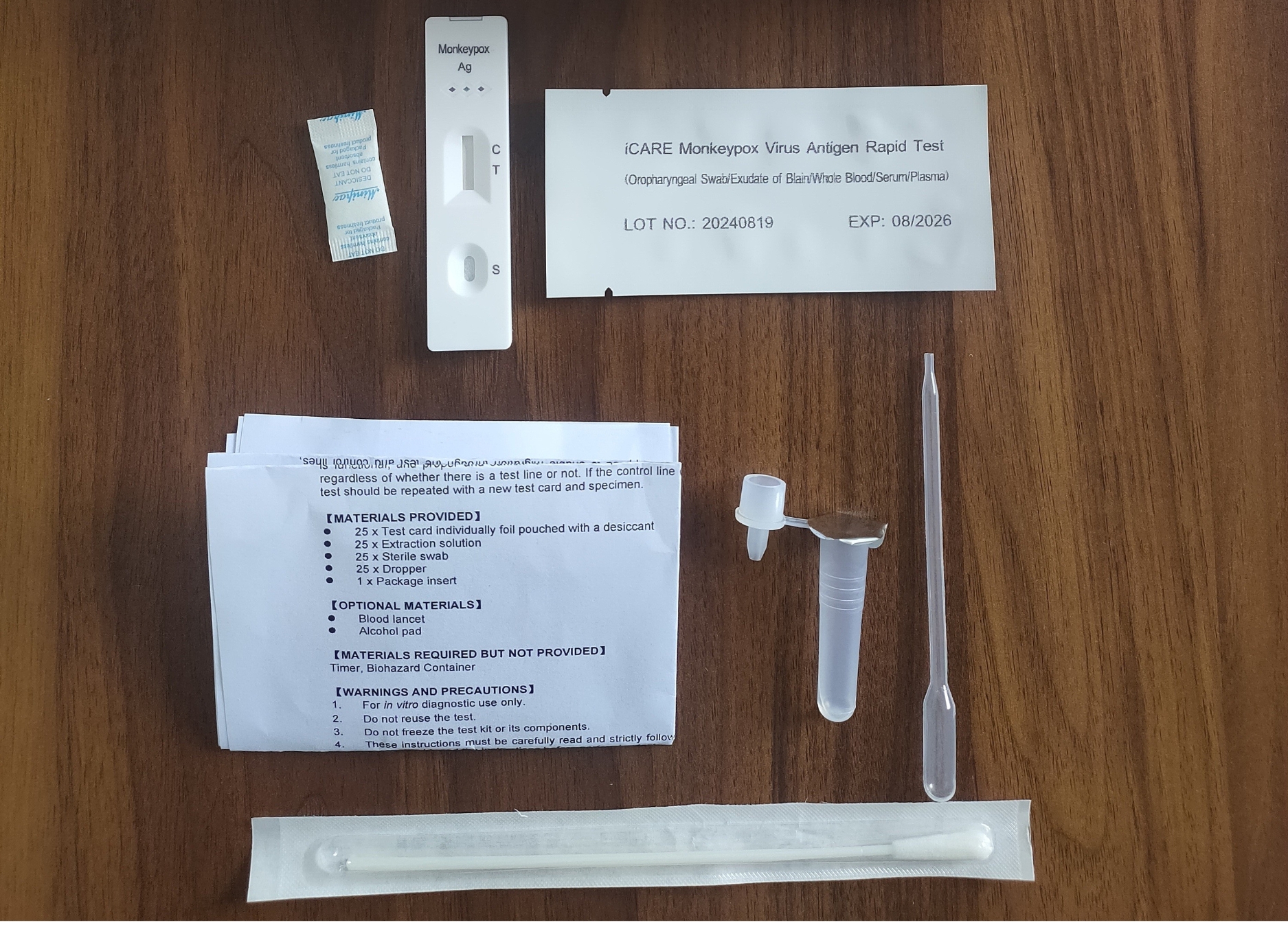
Monkeypox Virus Testing Procedure
Depending on the type of specimen used, the procedure varies slightly. Below is an overview of the steps involved:
Whole Blood/Serum/Plasma Samples
- Place a drop of the specimen into the specimen well on the test card.
- Add two drops of the provided extraction solution to the well.
- Read the results after 15 minutes.
Oropharyngeal Swab/Exudate of Blain Samples
- After collecting the specimen, immerse the swab into the extraction solution tube, rotating it to release the antigen.
- Add two drops of the extracted specimen to the test card and read the results after 15 minutes.
Interpreting the Results
Positive: A colored line appears in both the control line (C) and test line (T) regions.
Negative: Only the control line (C) is visible.
Invalid: If no control line (C) appears, the test result is invalid, and the test should be repeated with a new device.
Performance Characteristics
The iCARE Monkeypox Virus Antigen Rapid Test Kit has demonstrated high accuracy when tested against PCR standards:
Sensitivity: 99.08%
Specificity: 99.67%
Overall Accuracy: 99.52%.
These performance metrics affirm that the iCARE rapid test provides reliable results, crucial for timely diagnosis and response during Monkeypox outbreaks.
Limitations of the Monkeypox Virus Antigen Rapid Test
Although the test offers high accuracy, there are a few limitations to consider:
- The test is for qualitative detection only and does not indicate the quantity of virus present.
- False negatives may occur if the virus titer is below the test’s detection limits or if the sample is collected during an early stage of the disease when antigens are not yet detectable.
- A negative result does not necessarily rule out infection, especially if symptoms persist. In such cases, further testing with PCR or other diagnostic methods is recommended.
Advantages over Traditional Testing Methods
One of the standout benefits of the iCARE Monkeypox Virus Antigen Rapid Test is its speed. Unlike traditional PCR testing, which can take hours or even days to deliver results, this rapid test kit provides answers within 15 minutes. This allows healthcare workers to make faster decisions regarding isolation, treatment, and contact tracing, essential steps in containing outbreaks. Additionally, the test’s ease of use means that it can be administered in a variety of settings, including clinics, hospitals, and even remote locations where access to sophisticated laboratory equipment is limited.
Storage and Stability
The test kit should be stored between 2-30°C and should not be frozen. Its shelf life is 24 months from the date of manufacture, making it a long-lasting resource for medical facilities. It is important to ensure that all components are at room temperature before use, and the test card must be used immediately after being removed from its foil pouch.
Applications in the Healthcare Setting
Given the increasing risk of Monkeypox outbreaks, the iCARE Monkeypox Virus Antigen Rapid Test Kit is invaluable in healthcare settings. It enables quick identification of Monkeypox virus infections, helping clinicians implement isolation measures and appropriate treatment plans swiftly. The test is particularly useful in regions with limited access to laboratory infrastructure or where PCR testing capacity is overwhelmed during an outbreak.
Conclusion
The iCARE Monkeypox Virus Antigen Rapid Test Kit offers a timely solution for rapid and reliable detection of Monkeypox virus antigens in a variety of specimen types. Its high accuracy, ease of use, and quick results make it an essential tool for healthcare professionals working to prevent the spread of Monkeypox in both endemic and non-endemic regions. With this kit, professionals can ensure better management of Monkeypox cases, providing rapid diagnosis and thereby aiding in the containment of outbreaks.
FAQs for Monkeypox Testing Kit
What is the iCARE Monkeypox Virus Antigen Rapid Test Kit used for?
It is used for the qualitative detection of Monkeypox virus antigens in various types of human specimens, aiding in the diagnosis of Monkeypox virus infections.
How long does it take to get results from the test?
Results are available within 15 minutes after specimen collection and processing.
What types of samples can be tested with the iCARE kit?
The kit can test whole blood, serum, plasma, oropharyngeal swabs, and exudate of blain.
How accurate is the iCARE Monkeypox Rapid Test?
The test has demonstrated a sensitivity of 99.08% and a specificity of 99.67%, making it highly reliable.
Can the test be used at home?
No, the test is intended for professional in vitro diagnostic use only and should be administered by healthcare professionals.

